
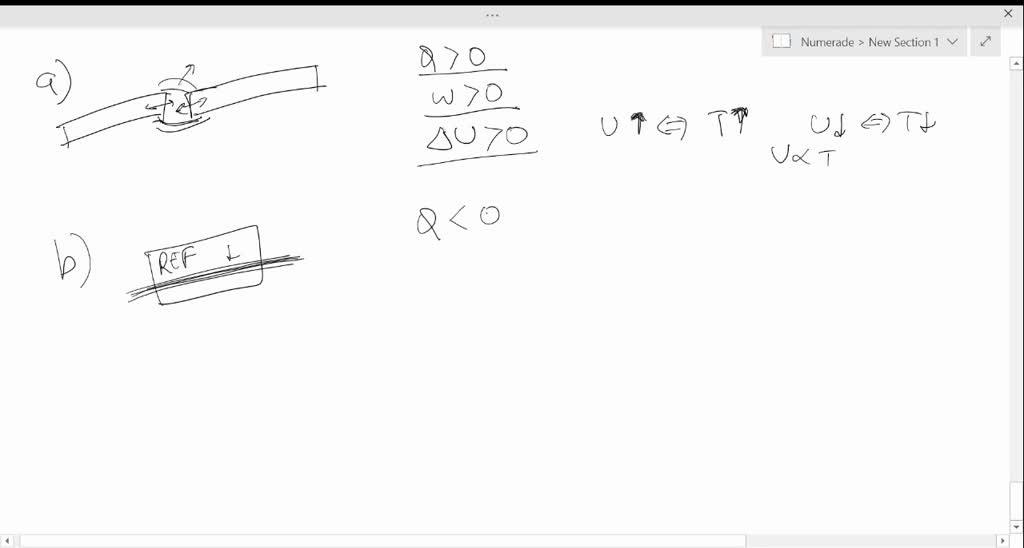

Necessary cookies are absolutely essential for the website to function properly. The system is usually defined as the chemical reaction and the boundary is the container in which the reaction is run. E > 0) when the system gains heat from its surroundings or when the surroundings do work on the system. When a system E is always negative?Į is negative when the system does work on its surroundings. Is exothermic positive or negative Delta E?Ī system that releases heat to the surroundings, an exothermic reaction, has a negative ΔH by convention, because the enthalpy of the products is lower than the enthalpy of the reactants of the system. Note that the value of ΔE is negative ΔE is negative for an exothermic reaction. This ΔE is the change in the chemical energy the reactants had 50 kJ more potential energy than the products did.
The relationship between the energy change of a system and that of its surroundings is given by the first law of thermodynamicsThe energy of the universe is constant: ΔEuniverse = ΔEsystem +ΔEsurroundings = 0., which states that the energy of the universe is constant. What is Delta E in the formula of 1st Law of Thermodynamics? The higher the Delta E value, the lower color accuracy. It is also the difference between two colors designated as two points in the CIELAB color space. What is Delta E? Delta E is used to ensure the color being displayed closely matches what the human eye receives.


 0 kommentar(er)
0 kommentar(er)
